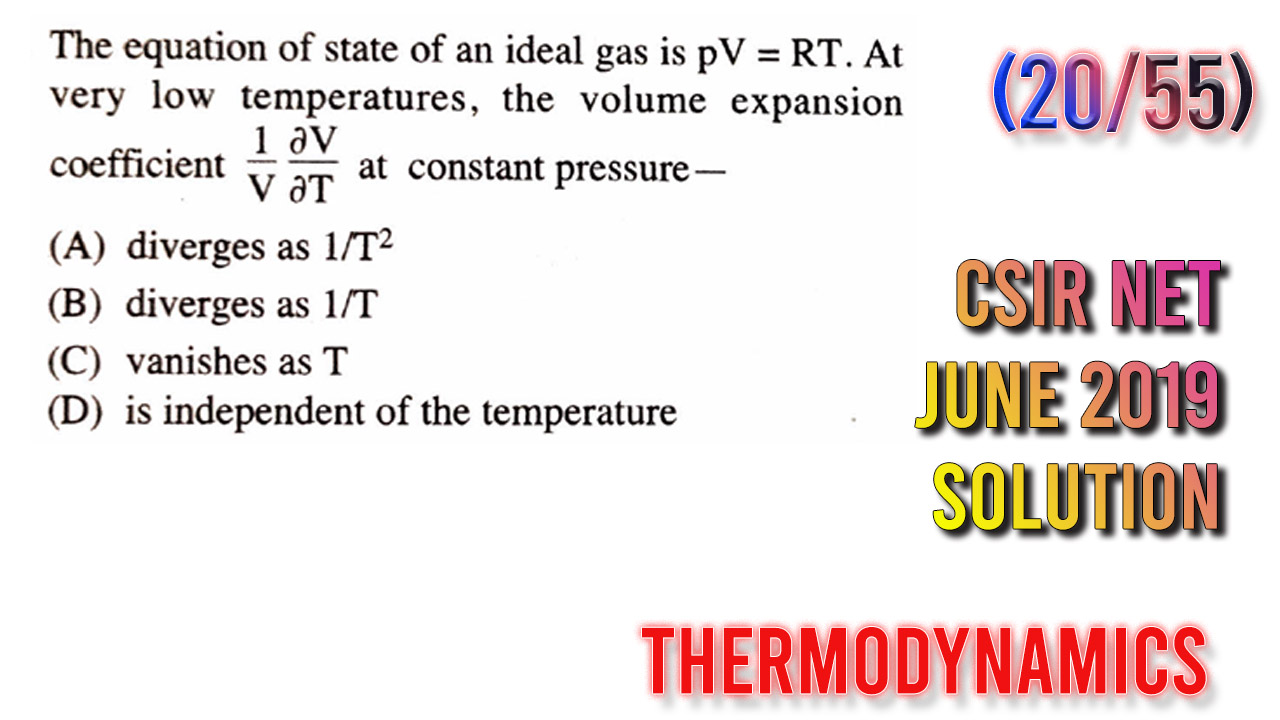
The question goes like this:
The equation of state of an ideal gas is PV = RT. at a very low temperature the volume expansion Coefficient at constant pressure…..

Solution is below
The Slide PDF can be found here :
Subscribe us 👇
Visit our parent website for more resources & Mock tests 👇